REGISTRATION FEES
| CDDF Industry partner representative (*) | Free |
| Non-commercial participant (academia/ foundations / charities) | Free |
| Representative from regulatory authorities (EMA, FDA, national agencies and HTA bodies) (**) | Free |
| Nurse & student | Free |
| Patient advocate (***) | Free |
| Pharmaceutical Company representative (not partners of CDDF) | EUR 615 (VAT- inclusive) |
| Miscellaneous – nonmedical, consultancy, freelance | EUR 615 (VAT- inclusive) |
(*) The list of CDDF Industry Partners is available here
(**) The representative from regulatory authorities’ rate applies to individuals who are employed by regulatory authorities such as EMA, FDA, national agencies and HTA bodies.
(***) The Patient advocate’s rate applies to individuals who are a member of a Patient Advocacy Organisation.
REGISTRATION PROCESS
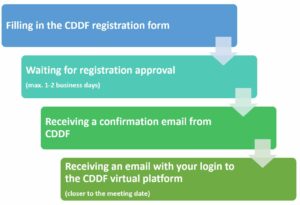
REGISTRATION DEADLINES
Free registrations
Registration deadline: Friday 5 February 2021
Paid registrations (Industry representatives from companies that are not CDDF Industry Partners)
- Registration deadline: Monday 1 February 2021
- Payment deadline: Friday 5 February 2021 (registration fees should be paid by the given deadline to complete paid registrations)
Cancellation
please note that only written cancellations addressed to info@cddf.org will be accepted with the following rules:
- Before 31 January 2021: 50% refund
- After 31 January 2021: no refund
CDDF Data Protection Policy
Please read the CDDF’s ‘Data Protection Policy.’