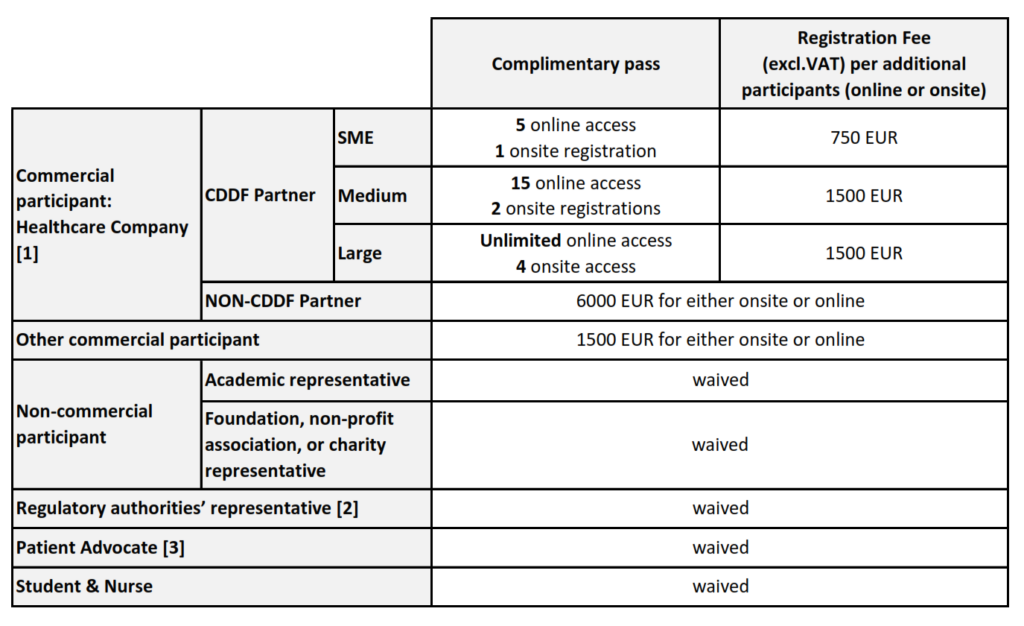
For more information about the registration fee structure, process and deadline, consult the information below.
REGISTRATION FEE STRUCTURE
[1] Is considered Health Care Company any company or commercial institution, or any division or subsidiary of any company or commercial institution, that is principally engaged in the health care industry, which shall include any types of health care business such as pharmaceutical companies; medical supply and equipment companies; technology, software, communications, financing and services vendors selling predominantly to health care companies; companies providing health insurance; clinical research organization and managed care companies.
[2] The representative from regulatory authority’ rate applies to an individual who is employed by a regulatory authority such as EMA, FDA, a national agency, and HTA body.
[3] The Patient advocate’s rate applies to an individual who is a member of a Patient Advocacy Organisation.
Registration fee includes:
- Virtual and/or in-person participation in the 3-day conference
- Access to the online workshop platform (recordings and slides available for 30 days after the conference)
- Coffee break on 6 February 2023 (in-person participants only)
- Lunch on 7 – 8 February (in-person participants only)
- Dinner on 6 – 7 February 2023 (in-person participants only)
Please note that accommodation and travel costs are NOT included in the registration fees for participants.
REGISTRATION PROCESS

![]()
REGISTRATION DEADLINE
Free registration
- Registration deadline onsite participation: Sunday 29 January 2023
- Registration deadline online participation: Friday 3 February 2023
Paid registration (see Registration fee)
- Registration deadline onsite participation: Sunday 29 January 2023
- Registration deadline online participation: Sunday 29 January 2023
- Payment deadline: Friday 3 February 2023 (registration fee should be paid by the given deadline to complete paid registration)
CDDF Data Protection Policy
Please read the CDDF’s ‘Data Protection Policy.’