REGISTRATION IS OPEN
To register, please fill out the form below.
For more information about the registration fee structure, process, and deadline, scroll down the page.
REGISTRATION FEE STRUCTURE
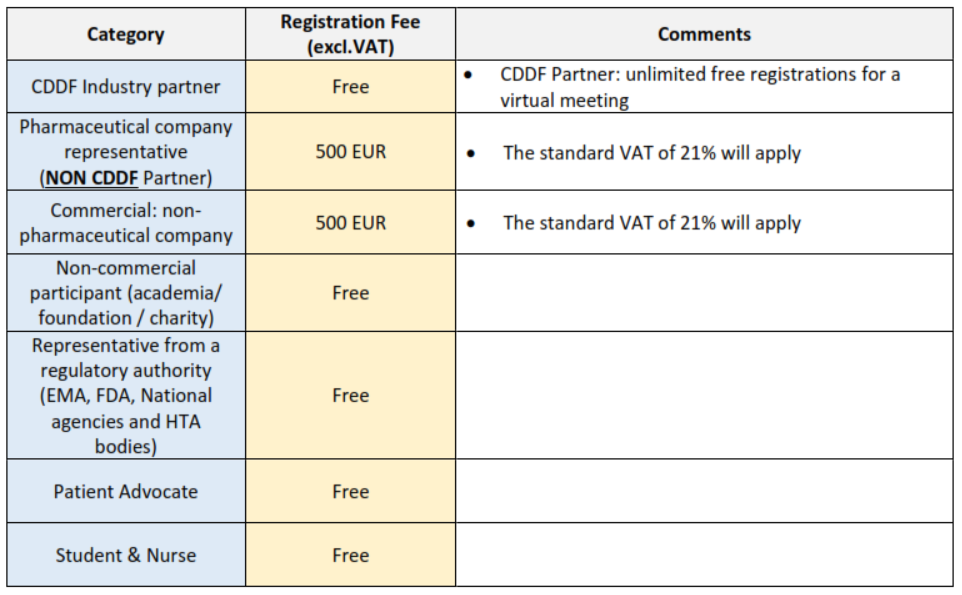
- The list of CDDF Industry Partners is available at the following link.
- The representative from regulatory authority’ rate applies to an individual who is employed by a regulatory authority such as EMA, FDA, a national agency, and HTA body.
- The Patient advocate’s rate applies to an individual who is a member of a Patient Advocacy Organisation.
REGISTRATION PROCESS
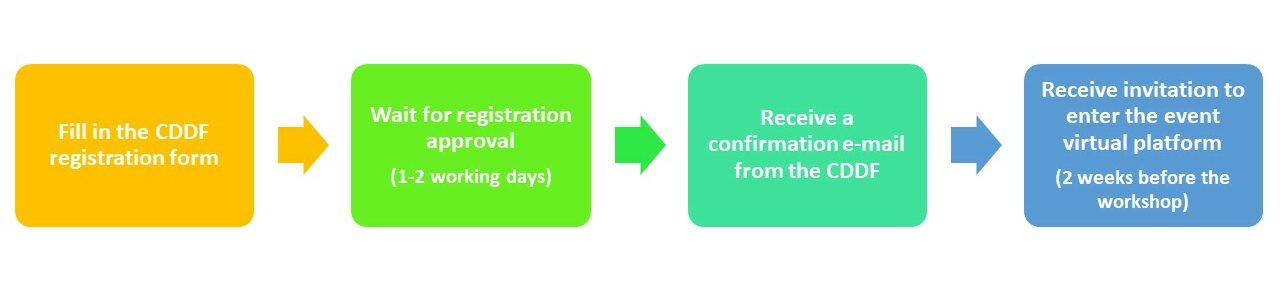
![]()
REGISTRATION DEADLINE
Free registration
- Registration deadline: Wednesday, February 2, 2022
Paid registration (see Registration fee)
- Registration deadline: Friday, January 28, 2022
- Payment deadline: Thursday, February 3, 2022 (registration fee should be paid by the given deadline to complete paid registration)
CDDF Data Protection Policy
Please read the CDDF’s ‘Data Protection Policy.’