CDDF joined forces with the Chinese Center for Food and Drug International Exchange (CCFDIE), the Center of Drug Evaluation (CDE) and the Chinese American Hematologist and Oncologist Network (CAHON) during the 2015 China Cancer Immunotherapy Workshop on June 23rd, 2015 in Beijing, China.
Cancer immunotherapy has progressed very rapidly in the past few years, with the potential to transform future new standard of care in oncology. Now the field of immunotherapy has joined the ranks of surgery, radiation, chemotherapy, and targeted therapy as a pillar of cancer therapy. Three new immune checkpoint agents have now been approved by the U.S. Food and Drug Administration (FDA) for the treatment of melanoma and lung cancer, and there is a high expectation that these agents, and others in this class, will also be approved over the next several years for treatment of patients with many other tumor types. In addition, chimeric antigen receptor (CAR)-T cell therapy and other immunotherapies have also shown remarkable progress and promise.
Building on the prior joint collaboration, the Chinese Center for Food and Drug International Exchange at CFDA (CCFDIE), and the Chinese American Hematologist and Oncologist Network (CAHON) are proposing a dedicated immuno-oncology annual meeting with the goal to educate and advance this rapidly evolving field to benefit the cancer patients in China, with the support from the Chinese Society of Clinical Oncology (CSCO), the Chinese Society of Immunology (CSI), and the Cancer Drug Development Forum (CDDF).
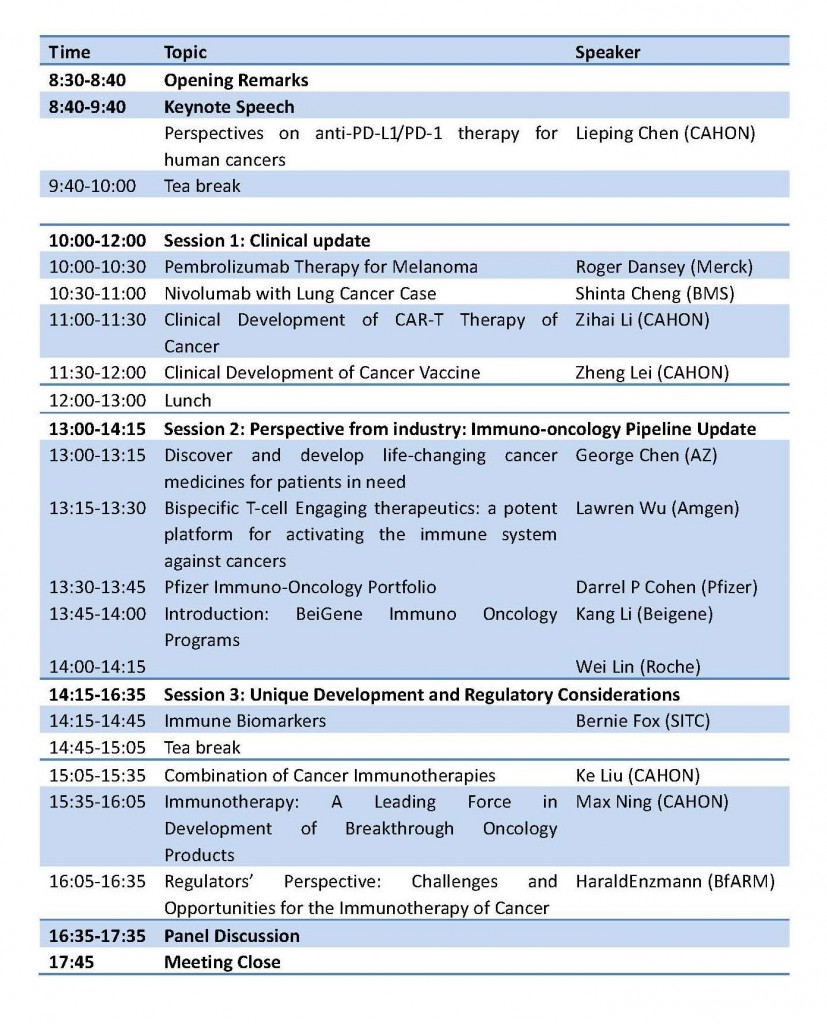
View the programme here below: