The CDDF Diversity Initiative is a CDDF-sponsored and directed project with a multi-stakeholder working group of regulators, payers, patients, academic researchers, and pharmaceutical companies.
This initiative aims to examine parameters of inclusion/diversity in clinical research, to support the planning and evaluation of clinical research, and the applicability of clinical trial outcomes to Europe’s diverse population.
Objectives
- To review and summarize the existing research, guidance, and data on variables that capture diversity and representativeness relevant for oncology clinical trials.
- To describe a set of variables relating to diversity and representativeness that can serve to plan and evaluate oncology clinical trials for their applicability to Europe’s population.
- To include the perspectives of key stakeholders: patients, healthcare professionals, academia, pharmaceutical industry, HTA views and medicines regulators.
- To consider the impact of such datasets on the planning/advice, conduct, and outcome evaluation of clinical trials, including for regulatory assessment / benefit-risk determination as well as HTA assessment.
 key deliverables
key deliverables
- To publish a consensus paper describing the selected variables, rationale for their selection, methodological approaches, and respective best practices for clinical trials and studies with the related stakeholder perspectives.
- To outline open questions for further research.
- To publish further scientific manuscripts on the discussed work packages
 Accompanying research
Accompanying research
- Qualitative/quantitative research and systematic review on possibly relevant diversity dimensions, to be completed as part of Phase 2.
- Expected Outcomes:
- White paper/Consensus paper after development of data item set in focus groups.
- Publication on feasibility of capturing diversity using the proposed data set in oncology.
 Benefits
Benefits
- A paper agreed upon by a cross section of key stakeholders setting out an approach and parameters for addressing EU patient diversity in oncology clinical trials and observational studies.
- In the absence of other guidance specific for the EU, it provides a reference point for developers, researchers and regulators when advising on, planning or conducting a clinical trial/study and when evaluating the clinical data produced in relevance to EU populations.
- It offers input to future guidance that may be developed at EU level on this topic.
 Project Outline
Project Outline
Preparation: Aug-Sep 2024
- Establish a co-ordinating committee
- Establish a working group
Phase 1: Oct 2024 - Jan2025
- Have a scoping discussion, including objectives and deliverables
- Outline what population variables are relevant to EU
- Clarify phase 2 objectives, budget, work packages and resource requirements
- Decide whether to proceed with Phase 2, or modify Phase 2
Phase 2: Feb 2025 - August 2026
- Establish collaboration with academic research group
- Define population descriptions relevant to oncology patients in the EU and what data elements and references can support these
- Prepare systematic literature review and publication
- Work on feasibility of capturing diversity using the proposed data set in oncology leading to a publication.
- Detect suitability of diversity data for use in regulatory decision-making
- Conduct work packages including meetings for topic discussions, workshop to present, and review consensus paper
- Finalise and publish consensus paper
- Close project
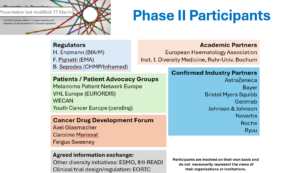
 Organization Committee & Working Group
Organization Committee & Working Group

Organization Committee
-
- Axel Glasmacher (CDDF)
- Fergus Sweeney (CDDF)
- Harald Enzmann (BfArM)
- Karin Kastrati (WECAN)
- Marie Von Lilienfeld-Toal (Ruhr-Universität Bochum)
- Birgit Wolf (Bayer)
- Anja Schiel (Noma)
- Sushmita Sen (Roche)

Working Group Phase II