

Gain valuable perspectives from CDDF participants and get an inside look at the CDDF Multi-stakeholder workshop on Biomarkers in Precision Oncology (Nov 2023)
Take a look at the interviews with participants from the CDDF Multi-stakeholder Workshop on “Biomarkers in Delivering Drug Development-Related Precision Oncology (13-14 November 2023, Amsterdam) and gain valuable perspectives from diverse stakeholders. Collaboration...
NEW PUBLICATION-WHITE PAPER: Histology Independent Drug Development – Is This the Future for Cancer Drugs?
The Cancer Drug Development Forum (CDDF) is delighted to announce that the White Paper has been developed following its ‘Histology Independent Drug Development – Is This the Future for Cancer Drugs?’ workshop, which took place on 14-15th November 2022, in Amsterdam....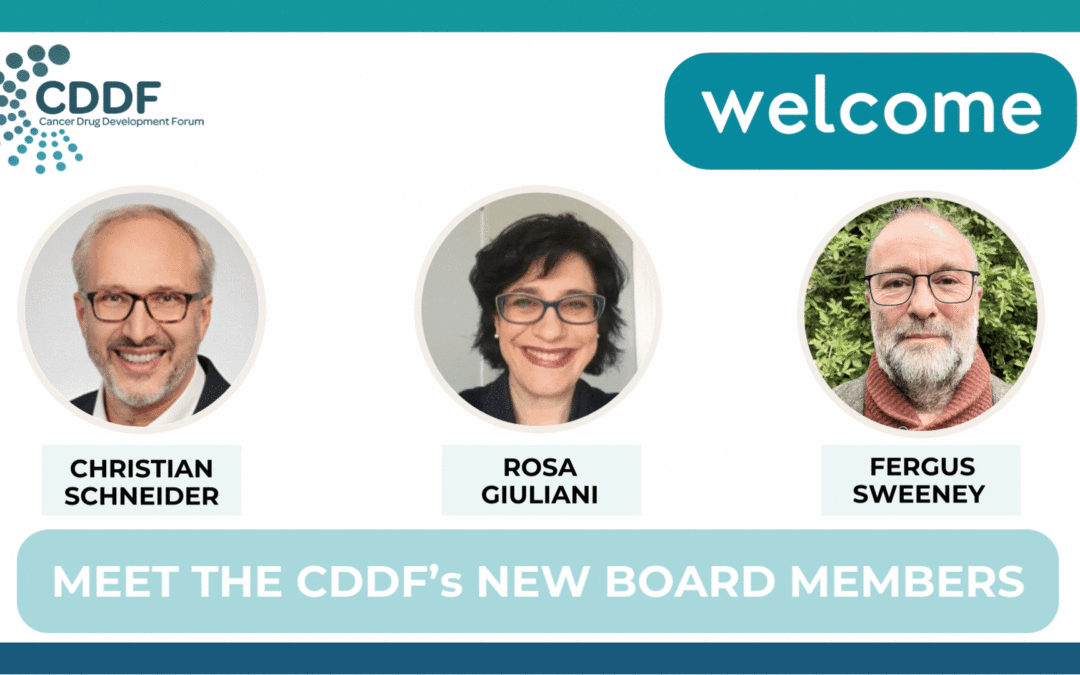
CDDF’s New Board Members: A Warm Welcome to Dr. Christian Schneider, Dr. Fergus Sweeney and Dr. Rosa Giuliani
With great pleasure, the Cancer Drug Development Forum (CDDF) announces the appointment of Dr. Christian Schneider, Dr. Fergus Sweeney, and Dr. Rosa Giuliani to its Board of Directors for a three-year term, effective 5 December, 2023. We warmly welcome these new...